Vinylic halides may be converted to grignard reagents by reaction with magnesium and these reagents undergo the same types of reaction as those derived from alkyl halides.
What are vinylic halides and alkyl.
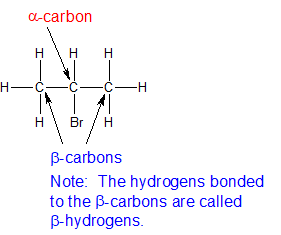
It is assumed that the alkyl halides have one or more beta hydrogens making elimination possible.
From the perspective of applications the dominant member of this class of compounds is vinyl chloride which is produced on the scale of millions of.
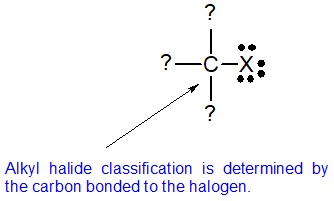
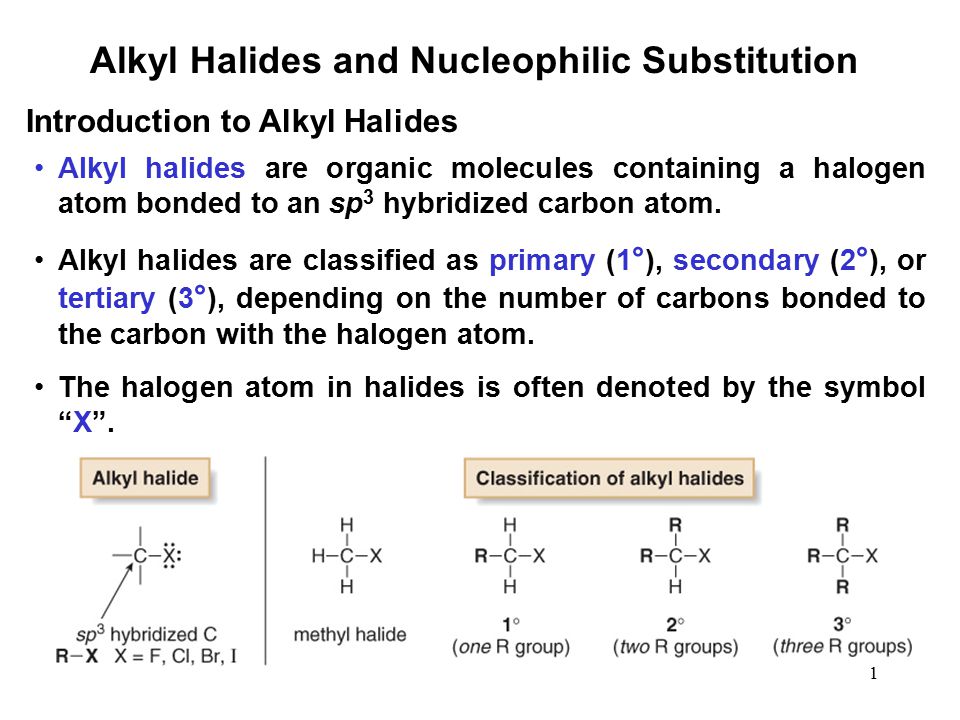
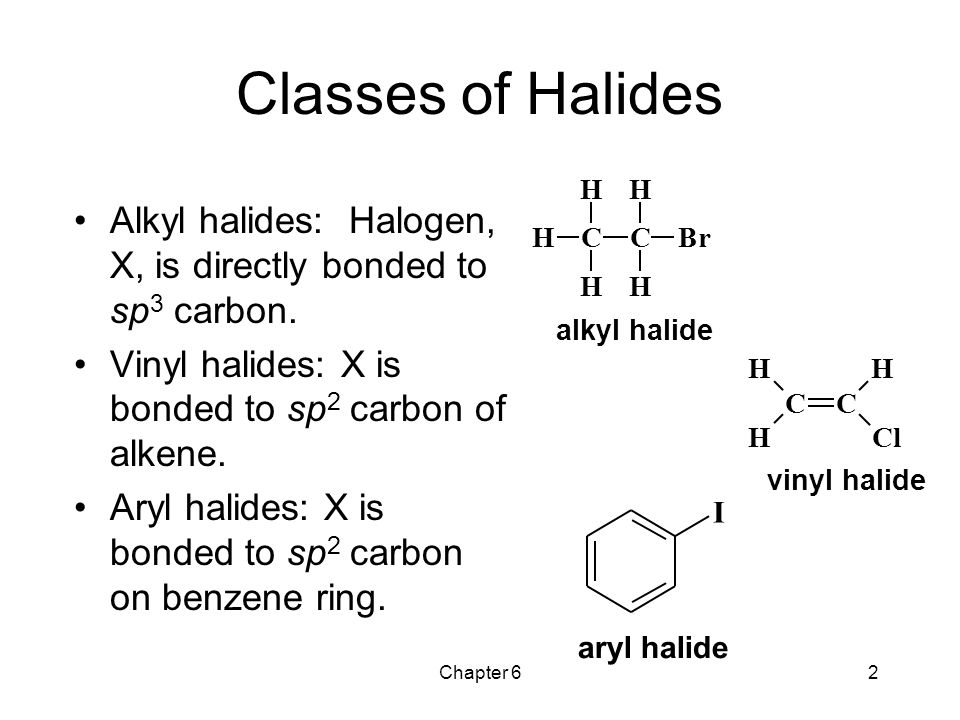
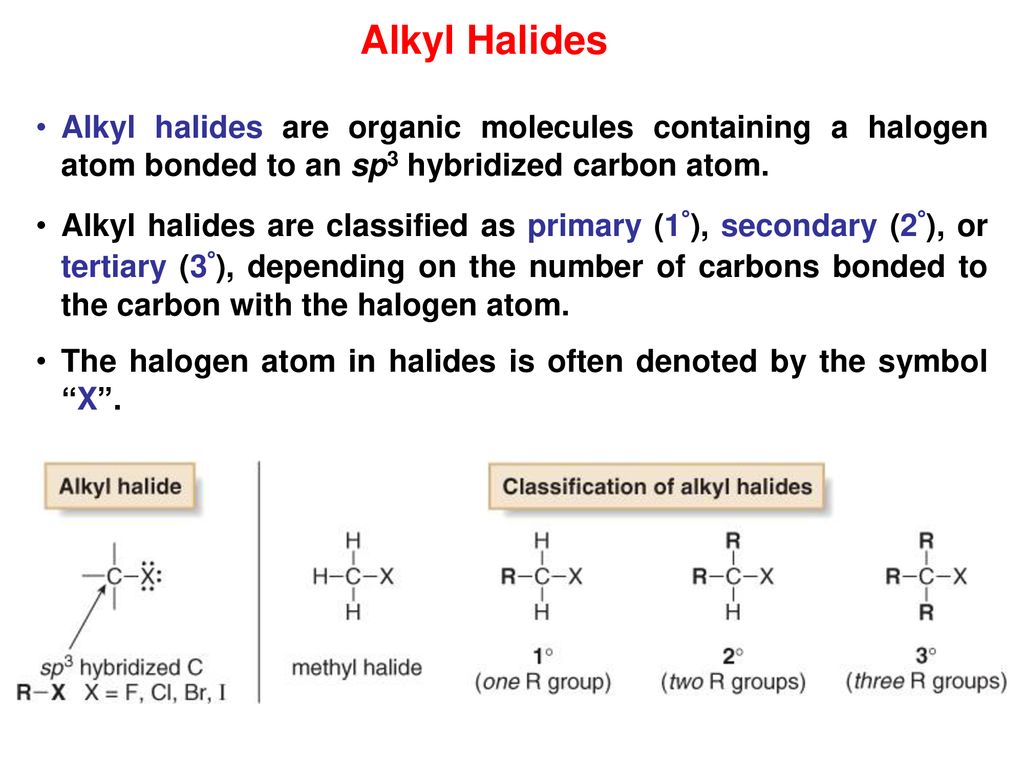
In alkyl halides all four bonds to the carbon that bears the halogen are single bonds.
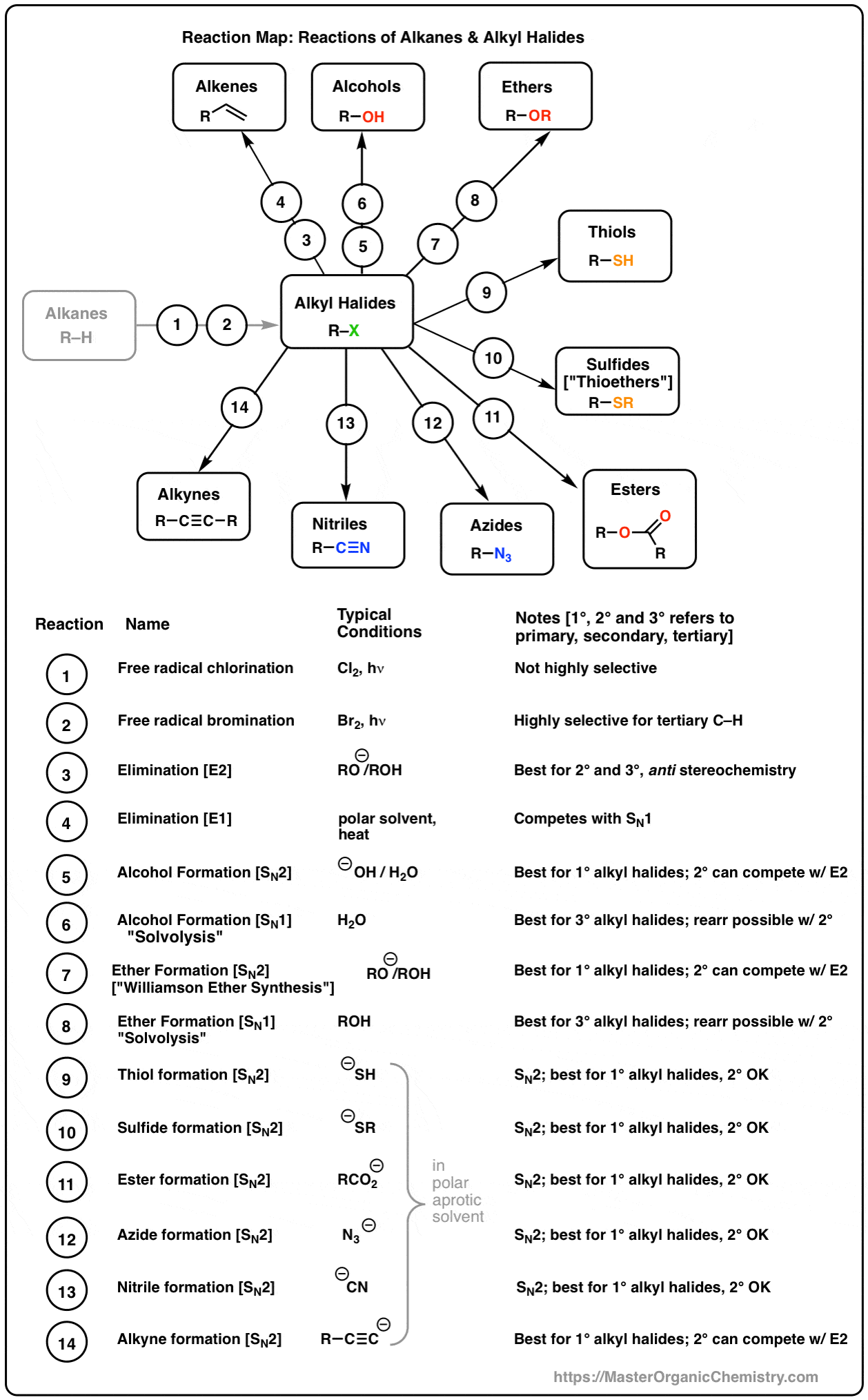
The following table summarizes the expected outcome of alkyl halide reactions with nucleophiles.
Firstly if the nuclophile comes in on the s n 2 path it will bump into a hydrogen or other group which is trans to the leaving group.
A vinyl halide is clearly a species with a formula h 2c c x h in which a halide is directly bound to an olefinic bond.
For this reason alkenyl halides with the formula rch chx are sometimes called vinyl halides.
An aryl halide has general formula c 6h 5x in which an halide group x has substituted the aryl ring.
And that low dielectric solvents e g.
Acetone ethanol tetrahydrofuran ethyl acetate are used.
Vinyl chloride h 2c chcl is an example.
Formally this is ethylene h 2c ch 2 with one of the hydrogens substituted by a heteroatom.
In aryl halides the halogen bearing carbon is part of.
An example is the addition of hydrogen chloride to vinyl chloride to yield 1 1.
The student asked why do vinyl halides not do the s n 2 reaction my answer was that two reasons exist for why the vinyl halide will not react with a nucleophile.
In vinylic halides the carbon that bears the halogen is doubly bonded to another carbon.
They are subdivided into alkyl vinylic aryl and acyl halides.
Vinylic halides resemble alkenes in that they undergo addition to their double bond.