The food and drug administration fda regulations for the conduct of clinical trials which have been in effect since the 1970s address both gcp and hsp.
What does cro stand for in clinical trials.
Phase 1 cro clinical trials are first stage trials that cro companies conduct on people.
Good clinical practice gcp is an international quality standard which governments can then transpose into regulations for clinical trials involving human subjects.
Clinical trial representation allows representing basic characteristic of a clinical trial such as study sponsor study name size of the trial number of participants.
Learn more about forte s enterprise ctms oncore enterprise research.
Gcp follows the international council on harmonisation of technical requirements for registration of pharmaceuticals for human use ich and enforces tight guidelines on ethical aspects of clinical research.
It defines basic elements such.
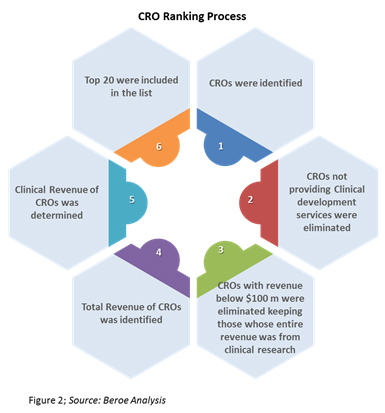
A contract research organization cro is a company that provides support to the pharmaceutical biotechnology and medical device industries in the form of research services outsourced on a contract basis.
Clinical trials reporting program.
Significant risk usually refers to device research.
A large scale clinical trial in which the safety and efficacy of an intervention are assessed in a large number of patients.
Clinical trial management system.
Several drugs in this phase carefully test how they act on specific targets and are a lot the role of phase 1 cro clinical trials in pharma testing.
Fda regulations and guidance documents.
A contract research organization or cro is an organization hired by a company in the medical field to manage the company s clinical trials and perform other tasks to help bring a drug or device to the market.
Cdisc bridg model is a unifying model of the domain of clinical research and research studies.
A cro can assist with monitoring auditing project management and more helping to ensure compliance and keep clinical trials.
Cro contract research organization a contract research organization cro is a team of clinical research professionals that offer independent third party oversight to sponsors during their clinical research trials.
The food and drug administration generally requires new drugs to be tested in phase iii trials before they can be put on the market.
Once a drug is cleared in pre clinical or phase zero testing the next stage is to evaluate its potential effectiveness and any potential side effects.